Peripheral Neuropathy
Schmerzen
Pathophysiology:
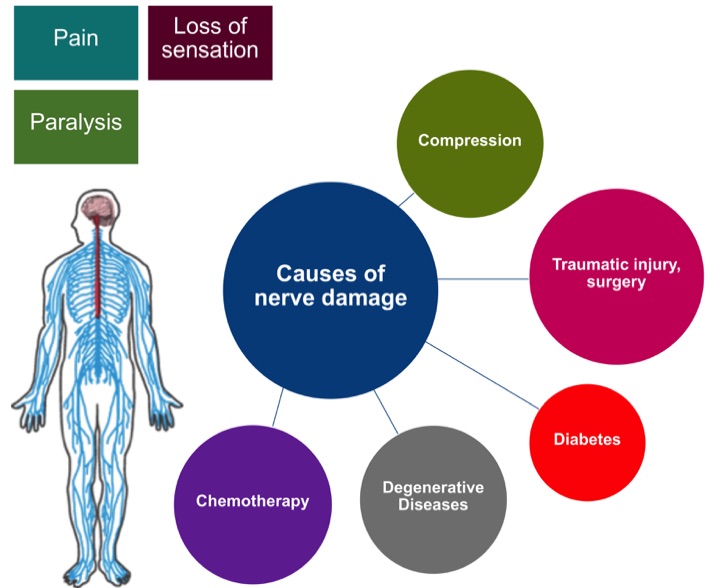
Nerve fibers, the so-called axons, conduct motor signals from the brain and spinal cord to their peripheral target organs like muscles. Conversely, sensory signals arrive via the peripheral nervous system (blue) from the skin to the spinal cord (red). Axonal damage to nerves (e.g., through compression, accidents, diabetes, chemotherapy) cause the interruption of nerve conduction and lead to corresponding declines in function like paralysis, loss of sensation, and chronic pain. A developing peripheral neuropathy significantly affects the patient´s quality of life and ability to manage everyday activities. Although injured peripheral nerves can regenerate in principle, functional recovery depends mainly on the spontaneous axonal regeneration rate. In particular, long regeneration distances are problematic due to the limited axonal growth rate of approximately 1-2 mm per day. This would mean a regeneration period of several months to years in cases of nerve damage to the legs and arms. Full recovery is often not possible as the long regeneration time can lead to irreversible damage to the target structures. Treatment is, therefore, very tedious and associated with high treatment and rehabilitation costs. Current therapy options are limited to surgical procedures (including nerve release, nerve suture or bypass) and conservative interventions such as physical therapy and electrostimulation. In addition to the regeneration distance, the type of damage, and the time of treatment also influence the success of regeneration. A clinically established therapy that can accelerate the growth of nerve fibers and thus qualitatively improve therapy outcome is not currently available.
The aim of our research is the development of new strategies to increase the regeneration speed of injured nerve fibers. This would not only shorten the recovery time but also prevent or reduce irreversible damage. A new active substance class showing these properties in animal experiments is currently under investigation by our department. The project is funded by the Federal Ministry for education and research (PARREGERON).
Chemotherapy:
In approximately one-third of cancer patients, certain chemotherapeutic drugs can lead to nerve damage. Nerves that are responsible for tactile sensitivity, temperature perception, nociception, and pain transmission are often affected, with patients developing a so-called polyneuropathy.
Chemotherapeutic drugs that frequently cause neuropathy in cancer patients mainly belong to the group of platinum compounds, the taxanes or the vinca alkaloids.
The mechanisms underlying the damage induced by chemotherapy are diverse, and the subject of current research. Depending on the cancer drug type, the nerve endings or the insulating myelin sheath around the nerve cells are destroyed, ion channels essential for signal transduction are altered, structural components of the cell such as the microtubules are affected, or the nutrient supply to the nerve cell is restricted.
Traumatic injury, surgery:
Traumatic injuries are the most common cause of neuropathies. The leading causes of traumatic nerve injuries are traffic accidents, violent impacts, and surgical interventions, for example, if the surgeon accidentally cuts or damages nerve tissue. The severity of nerve damage is clinically distinguished into five grades.
Grade 1: The continuity of nerve fibers (axons) is not interrupted, but a blockage of signal transduction occurs, for example, caused by intense pressure on the nerve (see also compression). The clinical term for this condition is neurapraxia.
Grade 2-4: The continuity of axons is lost, which leads to so-called Wallerian degeneration. The clinical term for this is axonotmesis. The further classification of severity grades 3-4 depend on how many of the surrounding nerve sheaths are also affected by the injury. Causes for Grade 2-4 lesions are sudden blunt trauma or stretch.
Grade 5: The nerve is completely transected, including all nerve sheath structures (neurotmesis). Causes are mainly sharp trauma, for example, by knives or broken glass.
The success of nerve regeneration is, among others, dependent on the severity of the injury. A spontaneous regrowth without surgical intervention is not possible if the nerve is completely transected.
Compression:
Compression nerve damage is categorized into acute (e.g., caused by hematomas or abscesses) and chronic compression injuries. The latter is caused mostly by long-lasting, external pressure on the nerve (e.g.,carpal tunnel syndrome).
The carpal tunnel is a tube-shaped anatomical compartment at the base of the palm through which the median nerves pass together with nine flexor tendons of the fingers. Narrowing of the carpal tunnel exerts pressure on the median nerve resulting in permanent pain, paraesthesia, and numbness of the fingers. The chronic pressure interferes with the blood flow of the nerve causing damage to the myelin sheath andultimately, the nerve fibers. If pressure persists, weakening of the hand muscles will occur (predominantly in the thenar eminence), finally resulting in the loss of fine motor skills.
Initially, treatment options include conservative interventions such as splints. If symptoms remain, carpal tunnel release surgery can be performed to relieve the median nerve from pressure. Concerning the severity of damage already present at the time of surgery, spontaneous regeneration of the median nerve takes place.
Diabetes:
In diabetic neuropathy, nerves are damaged because of permanently elevated blood sugar levels. Approximately every third diabetes patient develops neuropathy, which therefore belongs to the most frequent source of secondary damage of diabetes and is independent of the diabetes type.
The symptoms of diabetic neuropathy are very diverse. Pain perception, touch, or temperature sensitivity could be impacted, but patients may also develop chronic pain, abnormal sensation, and paralysis. In addition to the peripheral nervous system, the autonomic nervous system is frequently affected, which controls many functions of the body.
The molecular mechanisms that lead to the development of diabetic neuropathy are not yet fully understood and are the subject of current research. However, it is clear that permanently elevated blood sugar levels lead to vessel damage and thus, a reduced oxygen and nutrient supply for the nerves. Also, essential repair mechanisms and growth processes within nerve cells are disturbed, dramatically inhibiting normal regeneration.
Degenerative diseases:
Another cause of peripheral neuropathy are neurodegenerative diseases. A distinction is made between acquired and genetic diseases. Charcot-Marie-Tooth disease for example is a genetic degenerative disease of the PNS. The patients suffer from increasing muscle weakness and muscle wasting in the hands and feet, which are spreading further into arms and legs during disease progression. The disease causing mutations lead to direct damage of axons or the myelin sheath and thus disturb the innervation of muscles.
Autoimmune diseases are often the cause of acquired neuropathies. The neuropathy candevelop as a secondary disease, e.g. due to compression of the nerve caused by inflammatory joint diseases such as rheumatoid arthritis, or through a direct attack of the body's immune system on nerve structures as caused by Guillain-Barré Syndrome.
In addition, degenerative diseases of the central nervous system may also lead to the development of peripheral neuropathies for example the development of neuropathic pain in Parkinson´s disease.
Director: Prof. Dr. Dietmar Fischer

Center
for Pharmacology

